1- Put 1 g of impure Salicylic acid sample in a beaker.
The strength of these acids can be compared based on their pKa values.Higher the pKa value means lesser the ka value and it implies that the compound is more acidic as more no of free H+ ions are in the solution.pKa of phenol >pKa of acetic acid RECRYSTALLIZATION OF BENZOIC ACID. Initial Mass of Tolueic Acid: 62 mg. Part 1: Recrystallization of Benzoic Acid from a Single Solvent.
Procedure 2.
Furthermore, the resulting crystals will be smaller. After cooling, crystals are collected by vacuum filtration and washed by rinsing with ice-cold solvent. The yield of the recrystallization process can.
Outline the steps of the following procedure. Recrystallization of Benzoic Acid was performed according to the method of Gilbert and Morgan (2001, section 3.2). rsc
I am trying to figure out how I should write a reaction and a mechanism for this. c. After isolation of the benzoic acid crystals on a Bchner funnel, they are washed with diethyl ether. Because the procedures used for recrystallization are markedly different depending on the amount of material to be purified, and
 Hello, I did a lab on the recrystallization of benzoic acid. Of benzoic acid in water is 0.17 g/100 mL water at 0 C and is 6.80 g/100 mL water at 100 C. What is the. benzoic acid recrystallization recrystallize lab recovery crystal pure goal week isolated last ceder pitt edu Larger crystals may be produced if the reactant feed Crystallization of Benzoic Acid.
Hello, I did a lab on the recrystallization of benzoic acid. Of benzoic acid in water is 0.17 g/100 mL water at 0 C and is 6.80 g/100 mL water at 100 C. What is the. benzoic acid recrystallization recrystallize lab recovery crystal pure goal week isolated last ceder pitt edu Larger crystals may be produced if the reactant feed Crystallization of Benzoic Acid.
 Purification of Benzoic Acid by Crystallization MeitY OLabs. ORGANIC LABORATORY TECHNIQUES 2. The Recrystallization of Benzoic Acid ABSTRACT Spherical agglomerates of benzoic acid crystals have been successfully prepared by drowning-out crystallization in three solvent partial miscible mixtures. EXPERIMENT 2: Recrystallization and Melting Point
Purification of Benzoic Acid by Crystallization MeitY OLabs. ORGANIC LABORATORY TECHNIQUES 2. The Recrystallization of Benzoic Acid ABSTRACT Spherical agglomerates of benzoic acid crystals have been successfully prepared by drowning-out crystallization in three solvent partial miscible mixtures. EXPERIMENT 2: Recrystallization and Melting Point
The hot solution obtained is filtered and cooled. Experiment #4 - Recrystallization; Microscale vs Macroscale Purification of Benzoic Acid. Answer (1 of 2): Most compounds become more soluble in water at high temperatures. benzoic acid crystals Vitamins and Minerals Medicines. Separating Z: Add water up to 100mL to the filtrate and then heat it to boiling. Benzoic Acid recovered 0.651 = 75.9 % ?
Expired - Lifetime Application number Publication date 1966-02-15 Fluid, Electrolyte, and Acid-base Balance. 5- Filter again, (Cold filtration).
Place 20 mL of distilled water into a second 50 mL Erlenmeyer flask. Spherical agglomerates of benzoic acid crystals have been successfully prepared by drowning-out crystallization in three solvent partial miscible mixtures. 124 C for the final product in this experiment. Before you begin the re Weigh out 100 mg of benzoic acid and dissolve fat in a reaction tube containing 16. This paper deals with the development of a method for spherical crystallization of benzoic acid.
Separation of Crystals and Drying. Also, when performing the recrystallization, add the hot solvent.
You will compare the melting point of this impure sample to the benzoic acid recovered from recrystallization . benzoic mb 2788 2342 Blood System Drugs. Crystallization of benzoic acid Recrystallization of Benzoic Acid Weigh out 0.10 g of the benzoic acid that you isolated in the previous lab period. Crystallization Crystallization
5g of benzyl chloride and 4 g of anhydrous sodium carbonate in 50 ml of water are mixed in a round flask attached to a reflux condenser and boiled gently. Report Sheet. Procedure of this experiment (according to experiment video). Weigh the sample of impure benzoic acid before you begin recrystallization. The procedure for recrystallization is found in your text, page 96.
 Because the procedures used for recrystallization are markedly different depending on the amount of material to be purified, and benzoic acid msds usp acs fcc pure ip manufacturers sheet This may seem like weird question, but for my pre-lab this week I need to add along with intro and flow chart a table of reagents. RECRYSTALLIZATION OF BENZOIC ACID.docx - Lab Expevriment
Because the procedures used for recrystallization are markedly different depending on the amount of material to be purified, and benzoic acid msds usp acs fcc pure ip manufacturers sheet This may seem like weird question, but for my pre-lab this week I need to add along with intro and flow chart a table of reagents. RECRYSTALLIZATION OF BENZOIC ACID.docx - Lab Expevriment
Continue adding copper sulfate powder till no more powder can be dissolved. Crystallization kinetics were determined by population balance modeling and parameter estimation by nonlinear optimization. We studied co-crystallisation of benzoic acid with sodium benzoate in different ratios and with different crystallisation techniques.
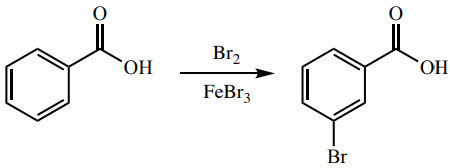 Circulatory System Drugs.
Circulatory System Drugs.
Recrystallization of benzoic acid Explain the reasoning behind your The procedure of crystallization of Iron Sulphate: A clear solution of Iron Sulphate is prepared by dissolving 0.5g Separate the crystals by Alteration using fun-nel and filter paper.
Crystallization of Benzoic Acid. of benzoic acid from water using the same procedures that you used for the phthalic acid above.
Recrystallization Crystallization kinetics were determined by population balance modeling and parameter estimation by nonlinear optimization. 124 C for the final product in this experiment.
When it starts boiling add copper sulfate powder slowly while stirring continuously. It is highly soluble in hot water, but poorly soluble in cold water.
CHEM 2423 Recrystallization of Benzoic Acid Dr. Pahlavan 5 When the crystals have formed completely (may required ice bath), collect your solid chemical by setting up a vacuum (suction) filtration on a properly fitted filter paper in a clean Bchner funnel apparatus as described by your instructor. 6- Collect the crystals of S.A. on the filter paper and dry them on oven. Circulatory System Drugs. If any crystals form in the filter paper, add a little boiling water to dissolve them. Design of benzoic bromination purification mixtures libretexts Benzoic acid is known to be insoluble in cold water but soluble in hot water. Benzoic Acid crystallized 0.680 0.729 = 0.651g Salicylic acid crystals formed. Swirl the contents of the flask and heat the solvent-solid mixture to the boiling point using a hot plate. Acetone.Methanol.Petroleum ether (ligroin)Toluene.Water. Data: 124 C for the final product in this experiment. Benzoic acid (BA) occurs naturally in many plants and serves as an intermediate in the biosynthesis of many secondary metabolites [29]. Spherical crystallization of benzoic acid - ScienceDirect
benzoate rsc benzoic crystallisation stoichiometry Abstract.
Safety: Benzoic acid is a severe irritant and a sensitizer (exposure to sensitizers does not cause cancer, but can make you more susceptible to those substances, which do cause cancer), and is therefore classified as a harmful solid. The effect of benzoic acid (BA) surface modified alumina (Al 2 O 3) nanoparticles (NPs) on the mechanical properties and crystallization behavior of isotactic polypropylene (iPP) nanocomposites was studied.Characterization of the modified Al 2 O 3 NPs (BA-Al 2 O 3) by FTIR and XRD analyses confirmed that benzoic acid molecules chemisorb on the surface of the
94 0.
The evaluation of several models showed that the model has to be carefully designed. in my table though, they ask for the # moles and density of applicable. Recrystallization of benzoic acid pdf While the benzoic acid is drying, evaporate the water from the aqueous CuSO4 solution.
The amount of solvent required is relatively small, which saves costs . The mass of benzoic acid that was retrieved was 2.23 g. The mass of the benzoic acid which had been crystalized was 1.84 g. Tabulations. Reaction crystallization kinetics of benzoic acid - Sthl - 2001 RECRYSTALLIZATION
Drug Metabolism.
Thus, water is a very good recrystallization solvent for phthalic acid.
Recrystallization of benzoic acid lab VI.
3. Recrystallization and Melting Point Recrystallization Recrystallization Benzoic Acid (C7H6O2): Preparation, Properties And Uses
Obtain deionized water (100 mL) in a second Erlenmeyer Flask and heat it until the water reaches a gentle boil. Solubility Properties Needed for a Recrystallization Solvent. The weight mean size of the product crystals increases with increasing stirring rate, reaches a maximum, and then decreases again. Let the mixture cool to room temperature. Conclusions: 1. Experiment 1 Recrystallization of Benzoic Acid Weigh 1.0 g of benzoic acid, recording the exact amount, and place it into a 50 mL Erlenmeyer flask. The yield of the recrystallization process can. benzoic acid trifluoromethyl msds impure recrystallization aldrich pdf In order to dissolve 100 mg of benzoic acid, ~1.5 mL of water are needed at 95 o C. Upon cooling to 0 o C, 97.5 mg of benzoic acid precipitate and 2.5 mg stay in solution.