There are two tests for carbon dioxide.

Why does calcium hydroxide solution turn milky when calcium carbonate is added? The characteristic test for CO2, is checking that the limewater is milky.  CO2(g) + Ca(OH)2(aq) -----> CaCO3(s) + H2O(l)The white milky suspension/precipitate is caused by the formation of calcium carbonate and explains the limewater test for carbon dioxide.Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colourless as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate:CO2 + H2O ------> H2CO3H2CO3 +CaCO3 --------> Ca(HCO3)2Ca(HCO3)2 = calcium hydrogen carbonate which is soluble in water.This chemistry is important in understanding how hard water is formed and then lime scale is formed in kettles and hot water boilers.
CO2(g) + Ca(OH)2(aq) -----> CaCO3(s) + H2O(l)The white milky suspension/precipitate is caused by the formation of calcium carbonate and explains the limewater test for carbon dioxide.Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colourless as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate:CO2 + H2O ------> H2CO3H2CO3 +CaCO3 --------> Ca(HCO3)2Ca(HCO3)2 = calcium hydrogen carbonate which is soluble in water.This chemistry is important in understanding how hard water is formed and then lime scale is formed in kettles and hot water boilers.
As this solution evaporates, the reverse reaction occurs which results in the formation of stalagmites and stalactites. The carbon dioxide and limewater react to produce water in addition to the calcium carbonate.
Many reactions produce gases which can help identifythe mechanisms and products involved. CO2 + Ca(OH)2 ----> CaCO3 + H2OHowever this is not an in depth answer, there's much more going on and the reaction can also reverse. chemical dioxide carbon lime water gas co2 through changes physical pass carbonate nitrogen happen does ekshiksha
In this article, we have answered all the questions related to the reaction of lime water and .
The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water.
Checkout JEE MAINS 2022 Question Paper Analysis : Your Mobile number and Email id will not be published. This article was co-authored by Meredith Juncker, PhD. Yes, if the water is carbonated, then it most likely contains CO2.
endstream endobj 1630 0 obj <> endobj 1631 0 obj <>stream Her studies are focused on proteins and neurodegenerative diseases.
Enter your email address to follow this blog and receive notifications of new posts by email.
%PDF-1.6
%
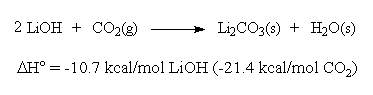
If it is oxygen, then the splint should relight. 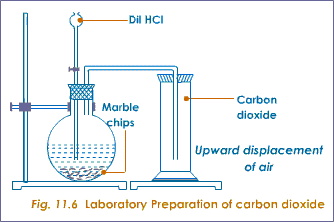 When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate.
When it reacts with sulphuric acid, it produces a cyan-blue colored chemical which is known as copper sulphate.
wikiHow marks an article as reader-approved once it receives enough positive feedback. I want to prepare copper sulphate using sulfuric acid. Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide.
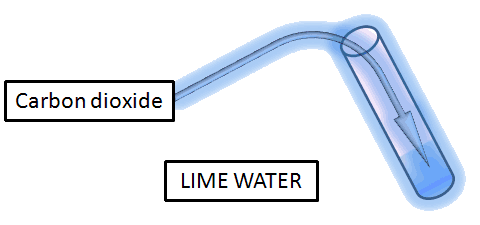
The other compound copper oxide is a compound that is formed when two elements copper and oxygen react with each other. If the limewater turns cloudy and milky white, your sample has carbon dioxide in it.
Good job, keep it up!".
Why do copper oxide and Sulphuric acid turn blue?
Approved. The sulfuric acid formula is . If necessary, repeat this filtering step until you obtain a clear limewater solution. Unlock expert answers by supporting wikiHow, https://earlieuk.wordpress.com/2011/02/18/how-to-collect-and-test-oxygen-hydrogen-and-carbon-dioxide/, http://www.docbrown.info/page13/ChemicalTests/GasPreparation.htm#Ex, http://chemstuff.co.uk/analytical-chemistry/tests-for-gases/, http://www.gcsescience.com/itestcarbondioxide.htm, https://sciencestruck.com/how-to-make-lime-water, http://mattson.creighton.edu/Download_Folder/MattsonGasBook4thEdCO2.pdf, http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel_pre_2011/chemicalreactions/preparinggasesrev4.shtml, http://antoine.frostburg.edu/chem/senese/101/inorganic/faq/co2-detection.shtml, Eseguire un Test per l'Anidride Carbonica.  If not then the gas which is subjected to the test is not carbon dioxide.
If not then the gas which is subjected to the test is not carbon dioxide.
Carbon dioxide extinguishes a lit splint: We can hold a small lit flame inside a test tube containing CO.
Store in a clean jar or bottle. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide.
wikiHow is where trusted research and expert knowledge come together.
You may be wondering what is lime water used for.  dioxide carbon test pearltrees
dioxide carbon test pearltrees
Being a weak base, copper oxide reacts with HCL easily to generate a soluble copper chloride and water. co2 dioxide carbon test molecule aq oh Capture the gas using a compressor, then cool the gas into a liquid.
This article has been viewed 307,784 times.
After this, you can use the tests above.
dioxide test carbon testing gases ks3 tes revision gas ks4 displays report Although there is no change in the effect, however, the nature of the split between t2g and eg orbitals in this new complex is such that it absorbs reddish-orange light. However, while it is a property of carbon dioxide, other gases, such as nitrogen, will also do this, so the test is not definitive and should not be quoted in an exam answer. reactive The appearance of this solid makes the liquid appear milky. Oxygen supports combustion so a good method of testing for oxygen is to take a glowing splint and place it in a sample of gas, if it re-ignites the gas is oxygen. Carbon dioxide is the only gas that turns lime water cloudy. Given this information, determine the activation energy for the reverse reaction, Er, and comment on the significance of the value (one sentence only).
If you don't want to boil anything, you can use a gas syringe to discharge the CO. Calcium hydrogen carbonate is soluble in water, making your solution clear!
Why are d-block elements not as reactive as s-block elements.
There are 8 references cited in this article, which can be found at the bottom of the page. The answer to this question is well known.
Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colourless. dioxide carbon test limewater milky lime water co2 turns diagram hydrogen chemistry splint presence science revision pop squeaky theres weebly
Copper(II)oxidereactswithsulfuric acidto create water andcopper (II) sulfate.
Thus, this is an unreliable test for carbon dioxide, and it may lead you to misidentify the gas.
is it a good thing using 2 named transition metals as examples, how reactive are transition metals compared to group 1, how to get pure water from salty water using household objects. 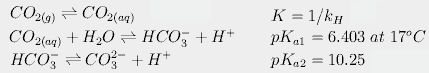
The equation of this chemical reaction is given below: The platform that connects tutors and students.
The byproduct of this reaction is sodium chloride (NaCl). someone help please, What is the boiling point of Styrofoam (polystyrene)? Copper sulphate takes on a bright blue colour. One of the most effective ways to test for carbon dioxide gas is the limewater test.
dioxide carbon andrews schoolphysics age16 thermal text Shake the solution vigorously for 1-2 minutes, then let it stand for 24 hours. 363 0 obj <> endobj You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. What is the maximum wavelength (in meters) of radiation capable of ionizing sulfur and producing this effect?
0 co2 carbonic acid dissociation bicarbonate equilibria warming cold facts global temperature randombio Put 1 teaspoon (4.9mL) of calcium hydroxide into a clean 1 gallon (3.8L) or smaller glass jar.
lioh carbon dioxide lithium hydroxide reaction water equations sides both
Fill the jar with distilled water.
To test for hydrogen a small sample can be ignited. Then, fill up a test tube halfway with the limewater and bring it to a boil. Now, we will answer how to test for carbon-dioxide. What salt is produced when copper oxide reacts with hydrochloric acid? Limewater and reaction results in a carbonic acid. Calcium carbonate is chalk, and when it is produced, it precipitates and solid particles of chalk appear.
carbon limewater sodium decomposition thermal dioxide test using cloudy hydrogencarbonate turns through baking heated powder gcse chemistry bbc bubbled detected Blow out the splint, then uncork the gas and place the splint inside while it is still glowing. This happens as the carbon dioxide forms acidic carbonic acid when it dissolves in the water, the carbonic acid (H2CO3) reacts further with the calcium carbonate. Measurements determine that photoelectrons associated with the 1st ionization energy of sulfur move with de Broglie wavelength =5.091 A. Hydrogen reacts very quickly with oxygen to form water. Now, the question arises why the solution turns milky.
Lime water turns milky as the Calcium hydroxide (chemical name for limewater) reacts with carbon dioxide to form Calcium Carbonate which is insoluble in water and thus forms a milky white precipitate.
Calcium carbonate is chalk, and when it is produced, it precipitates (i.e. If the gas is carbon dioxide, then the solution will turn milky.
The carbon dioxide and limewater react to produce calcium carbonate and water. Because of the higher reduction potential of copper as compared to hydrogen, it is unable to react with non-oxidizing acids like sulphuric acid and hydrochloric acid. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Bubbling carbon dioxide through the solution for an extended period of time makes the solution become clear and colorless.